Separation of Mixtures
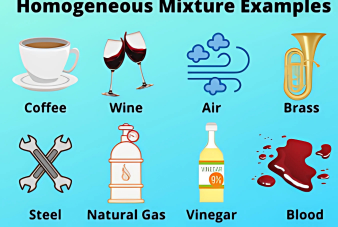
- Definition of Mixtures
- Types of Mixtures
- Reasons for Separating Mixtures
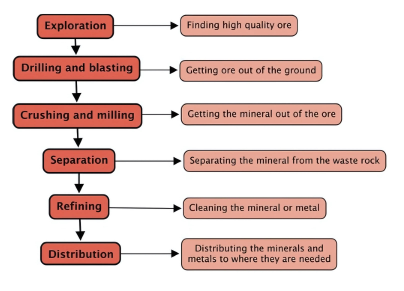
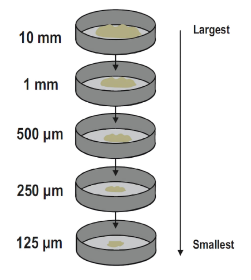
- Methods of Separating Mixtures
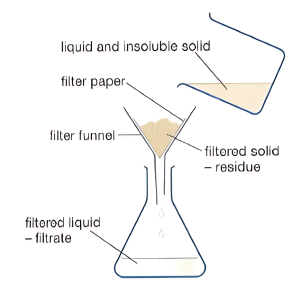
- Filtration
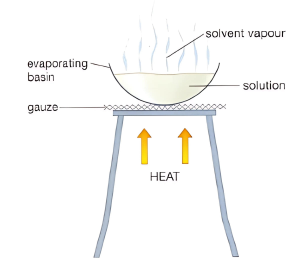
- Evaporation
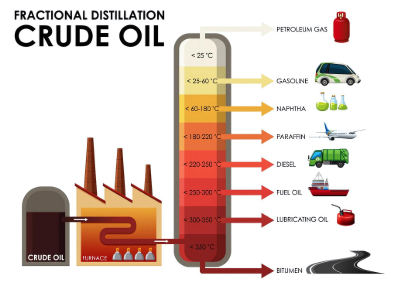
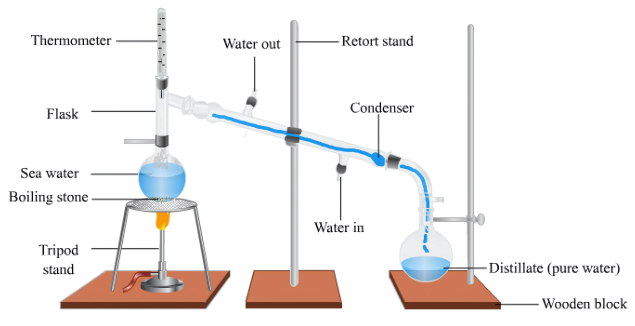
- Distillation
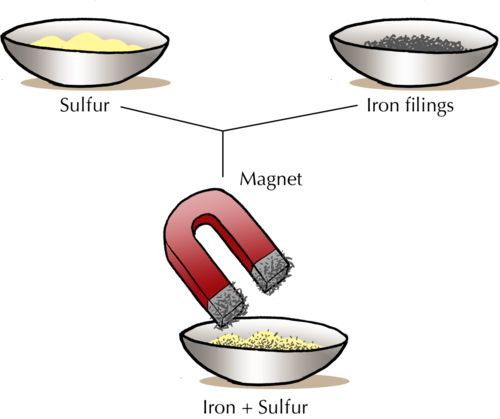
- Magnetic Separation
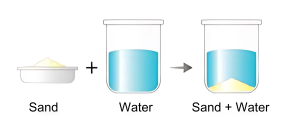
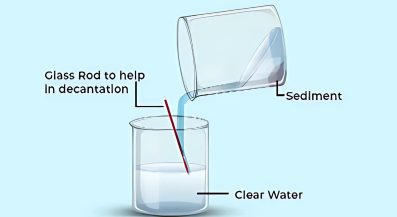
- Decantation
- Magnetic Separation of iron from sand
- Chromatography to separate the colors in ink
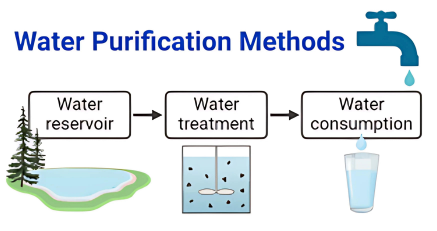
- Importance of Separation Techniques